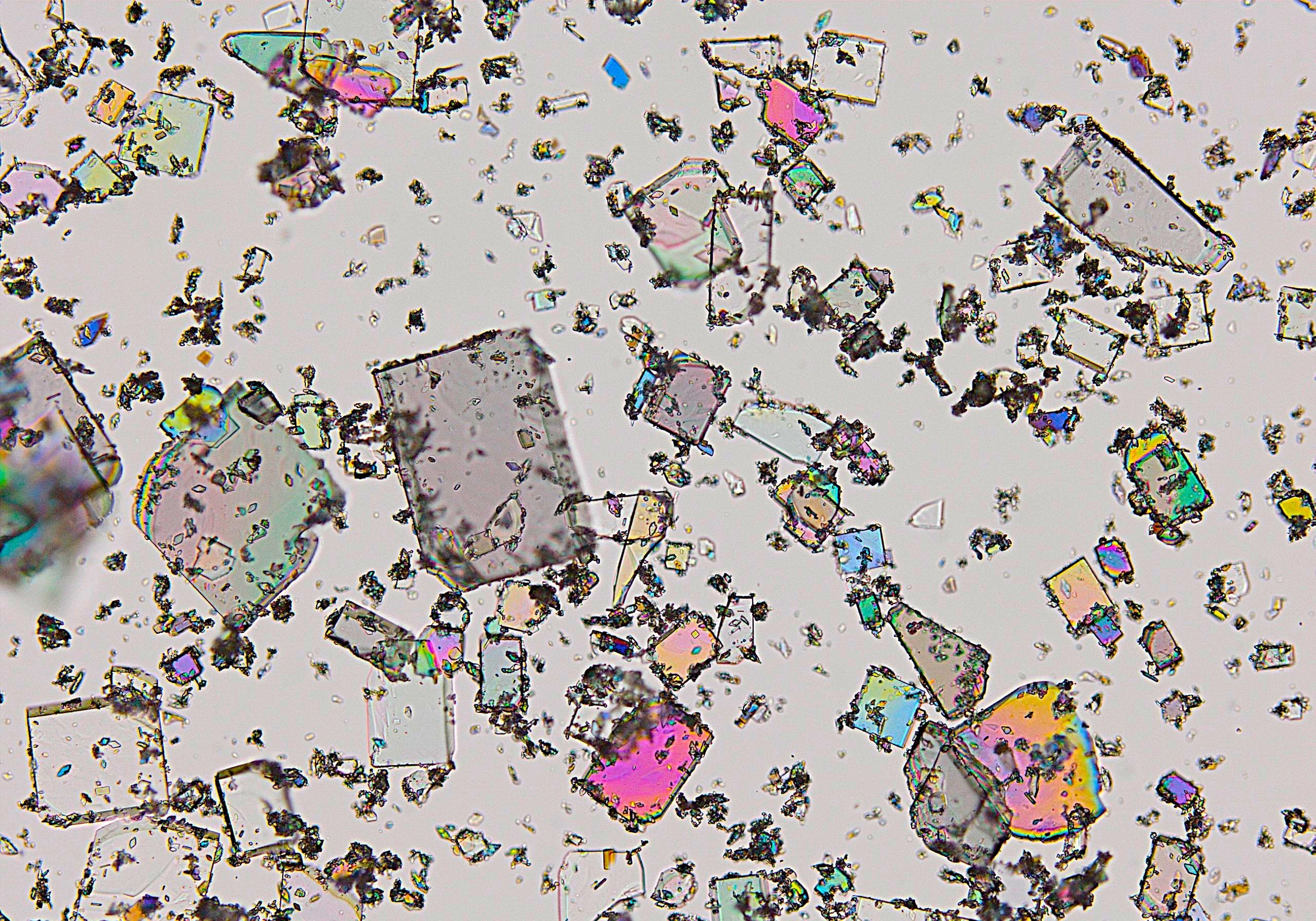
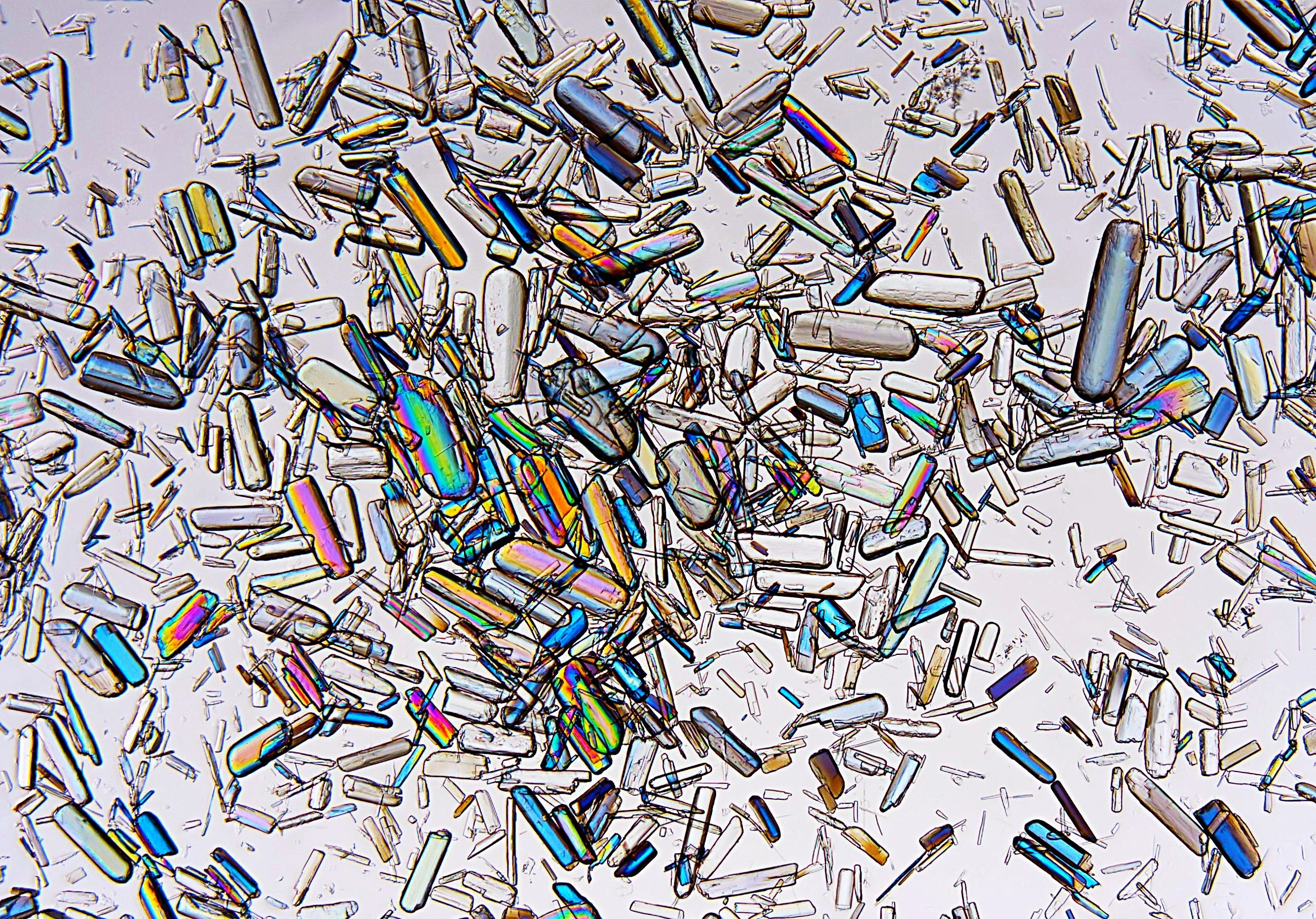
Process intermediates: Opportunities for improvements
Attention is rightly directed to establishing the desired attributes of the final material or drug substance which have been determined from the solid state investigations and the crystallisation process to enable development.